OPTIMA, the international research consortium, announces AstraZeneca and MUTABOR as official partners
AstraZeneca, the global biopharmaceutical company and MUTABOR, one of the biggest independent design agencies in Germany, have joined the international OPTIMA consortium. The two companies will complement the joint undertaking aiming to improve treatment for patients with prostate, breast and lung cancer through artificial intelligence.
Munich, Germany, 6th October 2022 – On 1st October 2021 the Innovative Medicines Initiative (IMI) – a joint undertaking of the European Union and the European Federation of Pharmaceutical Industries and Associations (EFPIA) – announced the launch of the public-private research programme OPTIMA (Optimal Treatment for Patients with Solid Tumours in Europe Through Artificial intelligence).
The € 21.3 million programme seeks to use artificial intelligence (AI) to improve care for patients with prostate, breast and lung cancer. OPTIMA’s goal is to design, develop and deliver the first interoperable, GDPR-compliant real-world oncology data and evidence generation platform in Europe, to enable healthcare professionals to provide the most optimal personalised care for each patient with solid tumours in the three cancers. Thirteen months after the start of the project, AstraZeneca and MUTABOR are now officially partners in the project.
The coordinators of the project, Prof. Dr James N’Dow from the European Association of Urology and Academic Urology Unit at the University of Aberdeen and Dr Hagen Krüger, Senior Medical Director Oncology, Pfizer Germany, welcome the addition of AstraZeneca and MUTABOR and the associated expansion of expertise within the project consortium.
Prof N’Dow, “On behalf of all partners I am delighted to welcome AstraZeneca and MUTABOR to the OPTIMA Consortium. Together with AstraZeneca and MUTABOR, OPTIMA will build on the strong foundation of all partners to ensure the project is at the forefront of driving the development and implementation of individualised patient-centric oncological care for prostate, breast, and lung cancer across Europe. All of the support and expertise to achieve this is gathered in OPTIMA, it is now time for us to work together in a highly collaborative atmosphere to achieve our ambitious objectives.”
Dr Krüger, « A broad-based public-private partnership of public partners and EFPIA companies is the best basis for uniting expertise and helping us achieve the vision of the OPTIMA consortium: improving cancer care for breast, lung, and prostate cancer patients through the use of artificial intelligence. EFPIA members are heavily engaged in the Innovative Medicines Initiative to fast-track collaborative research and boost pharmaceutical innovation in Europe, opening up new opportunities for cooperation from a biopharma point of view. »
As a global innovation and research-driven biopharmaceutical business, AstraZeneca focuses on the discovery, development and commercialization of prescription medicines for diseases with significant unmet clinical needs, including oncology. Its main contributions to OPTIMA will be towards the OPTIMA Platform implementation and evaluation (Work Package 5), together with EISBM and Amgen.
AstraZeneca will test the data platform for various types of users (e.g., clinicians, patients, data providers, bioinformaticians, sysadmins) and gather adaptation recommendations to be forwarded to ITTM and Amgen, which develop the platform.
David Dellamonica, Head of Digital and Innovation for AstraZeneca’s oncology business in Europe and Canada, “AstraZeneca is excited to become a member of the OPTIMA consortium. Gathering and – most crucially – learning from real-world patient data from across Europe is fundamental for achieving our shared ambition to revolutionise cancer care in Europe. By leveraging the latest technology to create tangible insights from this rich source of data, we will strengthen shared decision-making and establish standardised, evidence-based treatment protocols. Our ultimate aim is to give more people with cancer the chance to benefit from the latest medical and scientific innovations.”
In addition to the extended support from the pharmaceutical industry, the OPTIMA consortium is pleased to welcome the agency for design and transition, MUTABOR. MUTABOR will be responsible for the design of the OPTIMA platform and the extension of the project’s brand experience.
Burkhard Müller, Chief Digital Officer of MUTABOR, “At MUTABOR our goal is to drive positive change through design. Therefore, we are honoured to serve along with some of the leading universities, companies and scientists to contribute to OPTIMA. We add a user-centric mindset by understanding the needs of users and society, reducing complexity in communication and user interaction.”
With the joining of AstraZeneca and MUTABOR, the OPTIMA consortium brings together 38 partners from across 13 countries. It consists of private and public stakeholders in the clinical, academic, patient, regulatory, data sciences, legal and ethical and pharmaceutical fields.
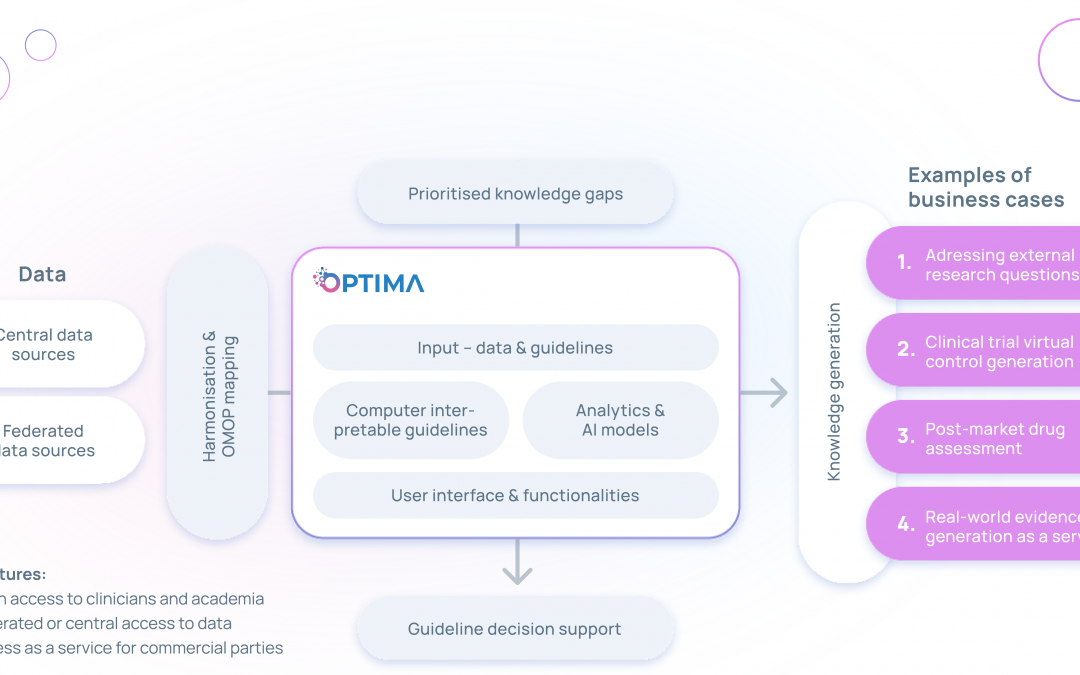
The interoperable OPTIMA platform will host datasets, data analysis tools, federated learning tools, AI algorithms and electronic decision support tools to identify, prioritise and fill the main knowledge gaps in prostate, breast and lung cancer – and propose improved clinical guideline recommendations. The developed AI-based decision support tools shall be employed in electronic health records (EHRs), thereby helping clinicians make care decisions based on the leading clinical practice guidelines for prostate, breast and lung cancer.
The platform could allow for the processing of high-dimensional data across sources and the use of deep learning to identify factors that enable individualised, real-time care decisions – ultimately providing personalised treatment for patients with the three cancer types. Through artificial intelligence and integrated next-generation tools and models, the consortium hopes that OPTIMA will be a key driver in the development of personalised care that recognizes each patient’s individual needs.
OPTIMA builds on other IMI projects (such as EHDEN, PIONEER and Harmony) that are supporting the European Health Data Space (EHDS), a European Commission initiative to promote better exchange and access to different types of health data to support healthcare delivery and health research and policy. If successful, OPTIMA could not only contribute knowledge and data to the EHDS but may also inform European policy regarding the clinical deployment of AI algorithms in healthcare.
Press contact
Verena von Scharfenberg, ARTTIC Innovation GmbH: press@arttic-innovation.de
Project office
Emma Jane Smith, European Association of Urology: e.smith@uroweb.org
Sigrid van Dorp, ttopstart: sigrid@ttopstart.com
OPTIMA is funded through the IMI2 Joint Undertaking and is listed under grant agreement No. 101034347. IMI2 receives support from the European Union’s Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA).
IMI supports collaborative research projects and builds networks of industrial and academic experts to boost pharmaceutical innovation in Europe.
The views communicated within are those of OPTIMA. Neither the IMI nor the European Union, EFPIA, or any Associated Partners is responsible for any use that may be made of the information contained herein.
OPTIMA Consortium Members*:
Abbvie
Amgen
Arttic Innovation
Association EISBM
Bayer AG
CASUS, Helmholtz-Zentrum Dresden – Rossendorf
European Association of Urology
European Cancer Patient Coalition
European Institute of Oncology
European Lung Foundation
European Organisation for Research and Treatment of Cancer
European Respiratory Society
Erasmus Universitair Medisch Centrum Rotterdam
German Cancer Society
GMV Soluciones Globales de Internet SAU
Helios Klinikum Emil von Behring
Institut de Cancerologie de l’Ouest
Institut Universitari d’Investigacio en Atencio Primaria
Information Technology for Translational Medicine
Ludwig-Maximilians-University Munich
Lund University
Maastricht University
Owkin
Oxford University
Queen Mary University of London
Pfizer
Roche
SmartReporting GMBH
The University of Tartu
The University Court of the University of Aberdeen
ttopstart
University College London
University of Vienna
Universita Vita-Salute San Raffaele
Uppsala University
YDEAL software